hankyoreh
Links to other country sites 다른 나라 사이트 링크
AI predicts COVID-19 treatments and vaccine will take between 2 to 5 years
It is generally understood that the development of a vaccine for an infectious disease takes 10 or more years. Mark Feinberg, president of the International AIDS Vaccine Initiative (IAVI), said in an interview with the health information website Stat News that the process could take 15 to 20 years.
Meanwhile, companies working to develop a vaccine for the novel coronavirus are setting a target of having a first vaccine out within the year. Three companies are currently in the clinical trial stage. China’s CanSino Biologics has advanced the fastest to Phase II trials, while Moderna and Inovio in the US are in Phase I trials. Can artificial intelligence (AI) predict when a COVID-19 vaccine and treatments will be available?
Clarivate, an internal academic information analytics company, accounted on Apr. 14 that an examination of the Cortellis AI biotech prediction system concluded that the world will have to wait two years for a treatment and five years for a vaccine. The timeline is shorter than the normal one for vaccine development, but far off from the target the companies have set. Clarivate explained that its projection was based on conditions as of Apr. 8, adding that the vaccine completion timeline could change depending on the progress made at predicted points.
Clarivate’s AI system predicted an availability date of October 2022 for the US company Gilead’s remdesivir, which is considered the leading candidate among treatments. Originally developed as a treatment for the Ebola virus, the drug is in Phase III clinical trial stage as a coronavirus treatment after it was discovered to be effective when used with COVID-19 patients. The AI system predicted an 89% likelihood of success with commercialization of remdesivir.
The “mRNA-1273” vaccine by Moderna Therapeutics, which began Phase I clinical trials in March, was predicted to complete Phase I and enter Phase II in 10 months. This vaccine encourages the formation of antibodies through the injection of messenger RNA (mRNA) with the genetic code for the spike proteins found on the virus’ surface. It’s Moderna’s first vaccine. The AI system projected an availability date of June 2025 for the Modern vaccine, with a 5% likelihood of success.
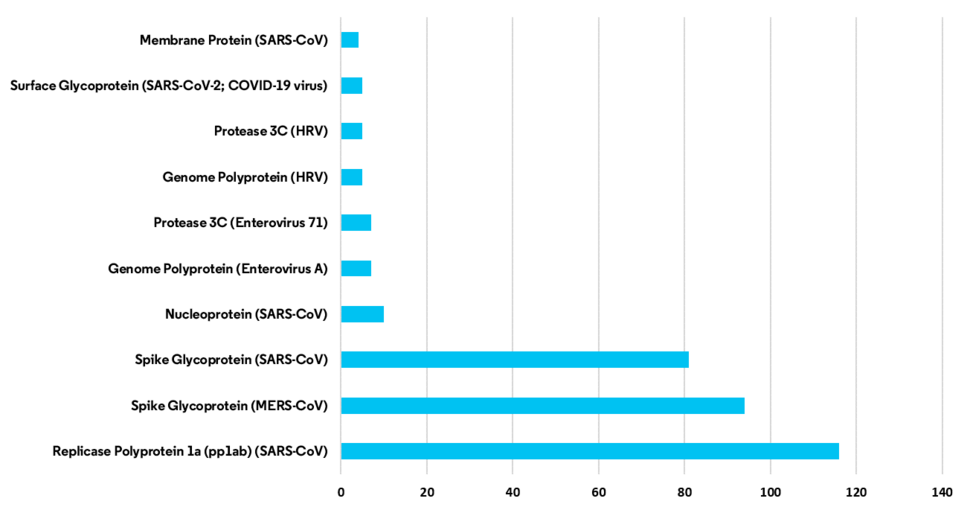
Clarivate explained that a total of 185 companies, research institutes, and universities were calculated as working on developing 156 total medications for the virus as of Apr. 8. By region, the US accounted for the most with 83, followed by China with 34. In development progress terms, 46% were at the candidate material stage, 42% at the preclinical stage, and 11% at the clinical stage, while 1% had halted their development efforts. This means that 88% are still stuck at the initial stage ahead of clinical trials. In South Korea, eight medications are under development, with two of them at the candidate material research stage and six at the preclinical stage, Clarivate said. No medications had yet reached the clinical trial stage.
Clinical trials are scheduled to begin in the third quarter of 2020 for a spike protein modulator monoclonal antibody under development by Celltrion, while Immunemed’s chimeric humanized virus suppressing factor received clinical trial approval in February. Enzychem Lifesciences previously announced plans to begin Phase I clinical trials in March for mosedipimod, the medication it is currently developing. Komipharm is working on repurposing the synthetic pharmaceutical KML-001, which it had been developing as a cancer treatment, into a treatment for the coronavirus. Bukwang Pharmaceutical is working on developing a hepatitis B virus treatment into a coronavirus treatment. Genexine and Binex are in the preclinical stage of development after signing a memorandum of understanding on co- development of a coronavirus DNA vaccine. SK Bioscience is conducting animal testing on candidate materials developed for a coronavirus vaccine.
By Kwak No-pil, senior staff writer
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press [Editorial] Penalties for airing allegations against Korea’s first lady endanger free press](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0502/1817146398095106.jpg) [Editorial] Penalties for airing allegations against Korea’s first lady endanger free press
[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press![[Editorial] Yoon must halt procurement of SM-3 interceptor missiles [Editorial] Yoon must halt procurement of SM-3 interceptor missiles](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/child/2024/0501/17145495551605_1717145495195344.jpg) [Editorial] Yoon must halt procurement of SM-3 interceptor missiles
[Editorial] Yoon must halt procurement of SM-3 interceptor missiles- [Guest essay] Maybe Korea’s rapid population decline is an opportunity, not a crisis
- [Column] Can Yoon steer diplomacy with Russia, China back on track?
- [Column] Season 2 of special prosecutor probe may be coming to Korea soon
- [Column] Park Geun-hye déjà vu in Yoon Suk-yeol
- [Editorial] New weight of N. Korea’s nuclear threats makes dialogue all the more urgent
- [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
- [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
- [Editorial] Japan’s rewriting of history with Korea has gone too far
Most viewed articles
- 1Months and months of overdue wages are pushing migrant workers in Korea into debt
- 2[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press
- 3Trump asks why US would defend Korea, hints at hiking Seoul’s defense cost burden
- 4Bills for Itaewon crush inquiry, special counsel probe into Marine’s death pass National Assembly
- 560% of young Koreans see no need to have kids after marriage
- 6Korean firms cut costs, work overtime amid global economic uncertainties
- 7[Guest essay] Maybe Korea’s rapid population decline is an opportunity, not a crisis
- 81 in 3 S. Korean security experts support nuclear armament, CSIS finds
- 9S. Korea discusses participation in defense development with AUKUS alliance
- 10[Reporter’s notebook] In Min’s world, she’s the artist — and NewJeans is her art