hankyoreh
Links to other country sites 다른 나라 사이트 링크
S. Korea has performed over 270,000 novel coronavirus tests in 2 months

As of Mar. 17, South Korea has tested over 270,000 people for the novel coronavirus throughout the past two months. The number is noticeably higher than the 138,000 tests performed as of Mar. 16 in Italy, which has experienced a steep rise in diagnoses. The cumulative number of patients who had tested positive as of the same day stood at 8,320 for South Korea and over 28,000 for Italy.
Indeed, while other countries have been scrambling to restrict arrivals from abroad, South Korean disease prevention officials have focused more on swift testing for those with apparent symptoms and populations with a high risk of cluster infections. Kwon Gye-cheol, chairperson of the Korean Society for Laboratory Medicine (KSLM), explained, “Once the virus has spread to a certain extent, it is not easy to stop infections simply by blocking overseas arrivals.”
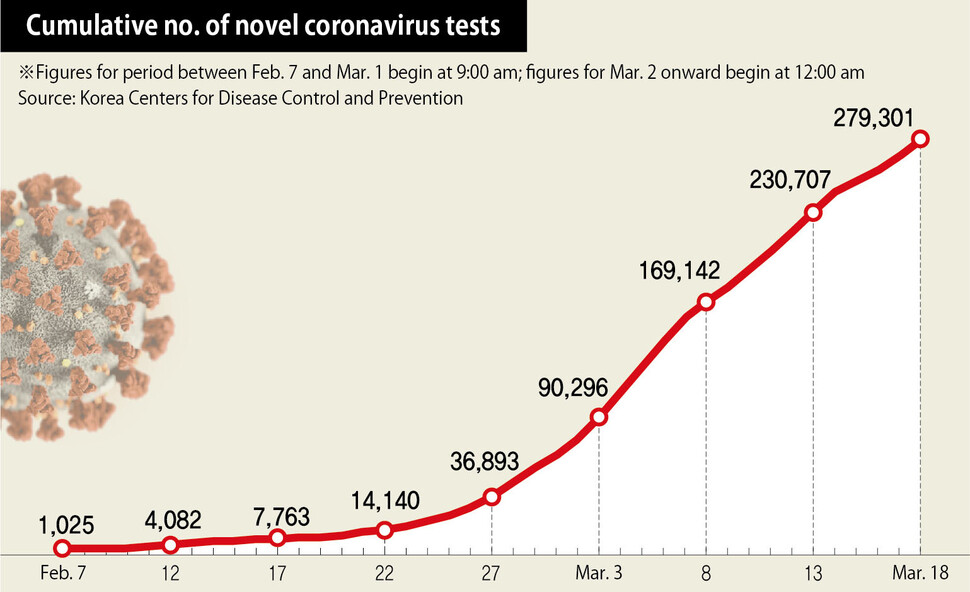
“The important thing is to enable early diagnosis and isolation by performing a lot of testing, and it appears that in South Korea’s case, this has had a positive impact in reducing the number of community infections,” he added.
The large volume of virus testing in South Korea is also why more asymptomatic patients have been identified than in other countries. In a recent briefing, Jung Eun-kyeong, director of the Korea Centers for Disease Control and Prevention (KCDC), said “In terms of us having a relatively higher percentage of asymptomatic cases than other countries, the large amount of testing has clearly been a factor, and this is going to be very helpful in understanding the illness’s epidemiological characteristics.”
How has testing evolved over the past two months?As recently as the early stages of the coronavirus outbreak in mid-January, it took over a day to produce a finding on whether someone was infected. This was because the primary approach was to use the pancoronavirus RT-PCR assay for detecting all known coronaviruses in a sample. Results took a long time because of the need to extract and amplify DNA from suspected patients and compare it with other coronaviruses. By Jan. 31, the process had been sped up by three to four times, reducing testing times to three to six hours. Public health officials had implemented real-time gene amplification testing approach known as polymerase chain reaction (PCR) based on the genetic sequences that had been identified. KCDC shared the testing method with the private sector, and the Ministry of Food and Drug Safety (MFDS) approved emergency use of a diagnostic kit incorporating it. Private businesses began producing kits one after the other.
The number of testing institutions able to process the tests also multiplied quickly. As recently as early February, only 20 or so national and local institutions were performing testing, including local Institute of Health and Environment branches. Currently, around 110 institutions are providing related services, including around 90 private testing institutions. Institutions were also given a type of “quiz” prior to being selected as an official testing center.
“As a test, public health authorities sent coronavirus samples to laboratories at each of the testing institutions. The institutions that answered correctly were selected as official testing centers,” explained Lee Eun-hee, director of GC Labs, which is currently performing coronavirus testing. Public health researchers were assigned to the task by the Institute of Health and Environment branches, while clinical pathologists and diagnostic medicine specialists were assigned by private testing institutions.
How to respond quickly in cases of cluster infections?On Mar. 10, a screening clinic was set up on the first floor of the Korea Building in Seoul’s Guro District to perform testing on visitors to the building and area residents after the Greater Seoul area’s largest cluster infection occurred there. The swift establishment of a large-scale screening clinic at a location that had been a key link in cluster infections enabled swift testing of suspected patients and individuals who had been in contact with infected persons. Another factor cited in South Korea’s fast and large-scale coronavirus testing has been the local governments’ disclosure of the locations visited by diagnosed patients, which has enabled citizens exposed to the risk of infection to quickly undergo testing at a screening clinic or elsewhere.
Possibility of error in coronavirus testing?The 20th patient diagnosed in South Korea had tested negative at first, and only received a positive diagnosis in another test performed after they had entered self-isolation. Experts identified three main reasons for the testing error. The first concerns cases where specimens have been taken inaccurately. To take a sample, medical staff apply a cotton swab to the back of the nasal cavity. A proper specimen may not be captured on the swab if the person undergoing testing flinches because of the pain. The second concerns cases where the virus’s quantity in a patient’s body is very low. When patients are asymptomatic or show few symptoms, the amount of virus emitted from their body is reduced. This may result in error even when normal testing methods are followed. The third scenario, which is seen as very unlikely, is that the testing itself was faulty. Experts place the likelihood of error with the current genetic amplification testing method for the coronavirus at around 1-2%.
Why are other countries taking notice?Since the Middle East Respiratory Syndrome (MERS) outbreak in 2015, South Korean public health authorities have been cooperating with the private sector while working to establish a “control tower” to supervise the infectious disease testing system. As a result, the government has been able to approve the emergency use of coronavirus diagnostic reagents, and cooperation has been facilitated, with biotech companies working quickly to produce items. Similar systems can also be found in the US and other countries, but none have been able to perform as many tests as South Korea amid the coronavirus pandemic. Testing institutions overseas have been unable to acquire all of the necessary reagents and equipment to meet the situation’s demands.
“My understanding is that testing is only being performed by the Centers for Disease Control (CDC) and a few other institutions in the US, and by a portion of major hospitals and infectious disease institutions in Japan,” said Kwon Gye-cheol. “That’s different from South Korea, where we have over 100 institutions doing testing.”
Jeon Chang-ho, a professor of diagnostic medicine at Daegu Catholic University Medical Center, said, “Other countries don’t have independent specialization programs in place for education specifically in diagnostic medicine the way South Korea does.”
“Over 1,000 people have graduated from this specialization program,” he noted.
Jang Cheol-hoon, a professor of diagnostic medicine at Pusan National University Yangsan Hospital, said, “Another factor making swift testing possible is the effective establishment of a public healthcare system, with testing costs at a much lower level than in places like the US or Japan.”
By Park Jun-yong and Seon Dam-eun, staff reporters
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0423/8317138574257878.jpg) [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
[Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father![[Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms? [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0423/3617138579390322.jpg) [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
[Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?- [Editorial] Japan’s rewriting of history with Korea has gone too far
- [Column] The president’s questionable capacity for dialogue
- [Column] Are chaebol firms just pizza pies for families to divvy up as they please?
- [Column] Has Korea, too, crossed the Rubicon on China?
- [Correspondent’s column] In Japan’s alliance with US, echoes of its past alliances with UK
- [Editorial] Does Yoon think the Korean public is wrong?
- [Editorial] As it bolsters its alliance with US, Japan must be accountable for past
- [Guest essay] Amending the Constitution is Yoon’s key to leaving office in public’s good graces
Most viewed articles
- 1[Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
- 2[Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
- 3Why Korea shouldn’t welcome Japan’s newly beefed up defense cooperation with US
- 4Opposition calls Yoon’s chief of staff appointment a ‘slap in the face’
- 5Senior doctors cut hours, prepare to resign as government refuses to scrap medical reform plan
- 6Terry Anderson, AP reporter who informed world of massacre in Gwangju, dies at 76
- 7New AI-based translation tools make their way into everyday life in Korea
- 8[Editorial] Japan’s rewriting of history with Korea has gone too far
- 9[Column] The clock is ticking for Korea’s first lady
- 10[Column] The president’s questionable capacity for dialogue