hankyoreh
Links to other country sites 다른 나라 사이트 링크
S. Korea to begin production of convalescent plasma medication for COVID-19
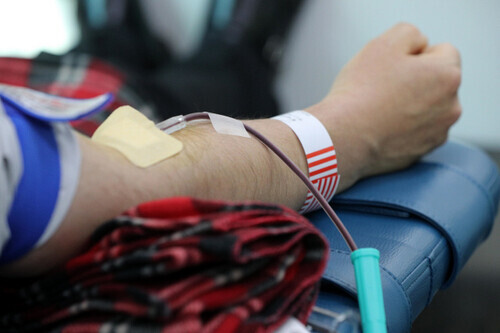
This week, production begins on a convalescent plasma medication for patients with severe cases of COVID-19, with the medication slated for use in clinical trials.
The medication is being jointly developed by the Korea National Institute of Health, under the Korea Centers for Disease Control and Prevention (KCDC), and GC Pharma, a private company.
“We have acquired the convalescent plasma that we need for developing the medication, and we will begin producing the medication on July 18 at the latest. Since we’ve received an exemption for the first stage of clinical trials, we’ll be able to move ahead with the second stage, which involves administering the medication to patients,” GC Pharma said on July 14.
As of Tuesday, 390 people had applied to donate blood, with 184 blood donors needed to develop the plasma medication.
The plasma method of treatment involves creating a medication with antibodies extracted from the blood of people who have fully recovered from COVID-19 and then administering that medication to patients currently suffering from COVID-19.
While numerous kinds of antibody-based medications are under development amid the COVID-19 pandemic, the development of plasma medication has been reportedly moving ahead quickly given the relative stability of this method of treatment. Last month, the South Korean government said its goal was to develop plasma medication before the end of the year.
COVID-19 patients will apparently be given this medication within the month. Since the therapeutic stability of plasma treatment has already been verified, researchers can skip the first phase of clinical trials and move straight into the second phase. Around a hundred individuals have reportedly been selected for this phase.
Researchers are speeding up efforts to acquire the additional blood they will need for commercializing the medication following the clinical phase.
The KCDC announced on July 13 that 500 members of the Shincheonji religious group who have recovered from COVID-19 will be donating plasma this week. As part of that effort, the KCDC will be collecting blood throughout this week in front of Kyungpook National University, in Daegu, using a plasma collecting vehicle on loan from the blood management office at the Korean Red Cross.
By Hong Seock-jae, staff reporter
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Column] When ‘fairness’ means hate and violence [Column] When ‘fairness’ means hate and violence](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0516/7417158465908824.jpg) [Column] When ‘fairness’ means hate and violence
[Column] When ‘fairness’ means hate and violence![[Editorial] Yoon must stop abusing authority to shield himself from investigation [Editorial] Yoon must stop abusing authority to shield himself from investigation](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0516/4417158464854198.jpg) [Editorial] Yoon must stop abusing authority to shield himself from investigation
[Editorial] Yoon must stop abusing authority to shield himself from investigation- [Column] US troop withdrawal from Korea could be the Acheson Line all over
- [Column] How to win back readers who’ve turned to YouTube for news
- [Column] Welcome to the president’s pity party
- [Editorial] Korea must respond firmly to Japan’s attempt to usurp Line
- [Editorial] Transfers of prosecutors investigating Korea’s first lady send chilling message
- [Column] Will Seoul’s ties with Moscow really recover on their own?
- [Column] Samsung’s ‘lost decade’ and Lee Jae-yong’s mismatched chopsticks
- [Correspondent’s column] The real reason the US is worried about Chinese ‘overcapacity’
Most viewed articles
- 1[Column] US troop withdrawal from Korea could be the Acheson Line all over
- 2China calls US tariffs ‘madness,’ warns of full-on trade conflict
- 3[Column] When ‘fairness’ means hate and violence
- 4Could Korea’s Naver lose control of Line to Japan?
- 5[Editorial] Yoon must stop abusing authority to shield himself from investigation
- 6Naver’s union calls for action from government over possible Japanese buyout of Line
- 7Several victims of Jeju Massacre still remain unaccounted for
- 8The quest to rediscover Jeju’s lost towns and villages
- 9Self-driving buses, taxis to hit roads in Seoul in October
- 10A Korean production studio may have made your favorite show – even if you don’t watch K-dramas