hankyoreh
Links to other country sites 다른 나라 사이트 링크
Kolon TissueGene was aware of risky cell alterations in treatment for 2 years
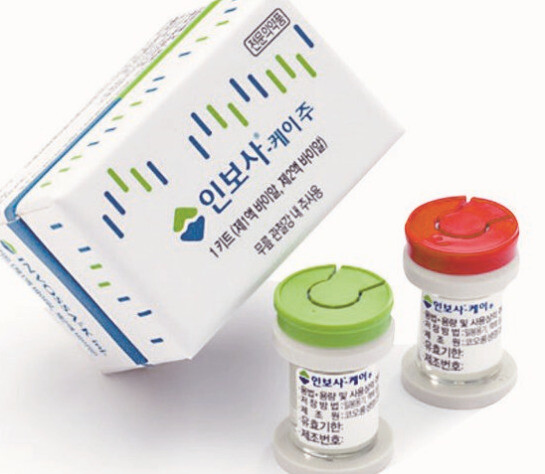
It has been revealed that Kolon TissueGene, a US subsidiary of Kolon Life Silence, knew for the past two years about a cell alteration in its Invossa-K treatment, the subject of a US investigation predicted to be opened this month. Some are now alleging that Kolon Life Science and public health authorities were also aware of the fact.
At the time Invossa received Ministry of Food and Drug Safety (MFDS) approval, the Solution 2 part of its two major components was identified as fast-growing cartilage cells; recently, the cell was found to be derived from the kidney (HEK 293). Kidney-derived cells reportedly carry a risk of tumor formation.
On May 3, Kolon Life Science issued an announcement stating that Invossa’s contracted producer Lonza was aware that Solution 2 consisted of HEK 293 cells following genetic screening during March 2017 testing of the solution’s production capability, adding that it had observed no problems in the production and notified Kolon TissueGene that it had been produced. According to accounts on May 6 from MFDS and Kolon, Lonza’s discovery that Solution 2 had been changed to HEK 293 cells rather than cartilage cells came four months before MFDS granted its approval of Invossa. This is the basis for accusations that Kolon Life Science failed to notify MFDS of the change despite being aware of it from the approval stage.
MFDS plans to take Kolon TissueGene’s awareness of the cell change as early as March 2017 into account as a basis for administrative action.
“The fact that Kolon TissueGene verified that it was HEK 293 is being viewed as a very serious issue,” an MFDS official said, adding that there would be a “comprehensive verification through a local US investigation scheduled to begin around May 20.” The MFDS previously ordered Kolon Life Science to submit related data by May 14.
Responding to accusations of giving a false explanation, Kolon Life Science maintained that it only became aware of the different cell components with the first genetic screening in late February.
“We are speculating that the Kolon TissueGene research team made the decision in 2017 not to report the issue higher up because they deemed the kidney cells not be a problem,” a Kolon Life Science official said.
“Our current understanding is that the facts were not reported to Kolon Life Science,” the official added.
By Kim Yang-joong, medical correspondent, and Hyun So-eun, staff reporter
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Editorial] Intensifying US-China rivalry means Seoul must address uncertainty with Beijing sooner than later [Editorial] Intensifying US-China rivalry means Seoul must address uncertainty with Beijing sooner than later](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0517/8117159322045222.jpg) [Editorial] Intensifying US-China rivalry means Seoul must address uncertainty with Beijing sooner than later
[Editorial] Intensifying US-China rivalry means Seoul must address uncertainty with Beijing sooner than later![[Column] When ‘fairness’ means hate and violence [Column] When ‘fairness’ means hate and violence](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0516/7417158465908824.jpg) [Column] When ‘fairness’ means hate and violence
[Column] When ‘fairness’ means hate and violence- [Editorial] Yoon must stop abusing authority to shield himself from investigation
- [Column] US troop withdrawal from Korea could be the Acheson Line all over
- [Column] How to win back readers who’ve turned to YouTube for news
- [Column] Welcome to the president’s pity party
- [Editorial] Korea must respond firmly to Japan’s attempt to usurp Line
- [Editorial] Transfers of prosecutors investigating Korea’s first lady send chilling message
- [Column] Will Seoul’s ties with Moscow really recover on their own?
- [Column] Samsung’s ‘lost decade’ and Lee Jae-yong’s mismatched chopsticks
Most viewed articles
- 1[Editorial] Transfers of prosecutors investigating Korea’s first lady send chilling message
- 2[Exclusive] Unearthed memo suggests Gwangju Uprising missing may have been cremated
- 3[Column] US troop withdrawal from Korea could be the Acheson Line all over
- 4[Column] When ‘fairness’ means hate and violence
- 5‘Shot, stabbed, piled on a truck’: Mystery of missing dead at Gwangju Prison
- 6S. Korea “monitoring developments” after report of secret Chinese police station in Seoul
- 7[Editorial] Intensifying US-China rivalry means Seoul must address uncertainty with Beijing sooner t
- 8US has always pulled troops from Korea unilaterally — is Yoon prepared for it to happen again?
- 9China calls US tariffs ‘madness,’ warns of full-on trade conflict
- 10Seoul government announces comprehensive measures to prevent lonely deaths