hankyoreh
Links to other country sites 다른 나라 사이트 링크
S. Korea in talks to purchase additional Pfizer COVID-19 pills following FDA approval
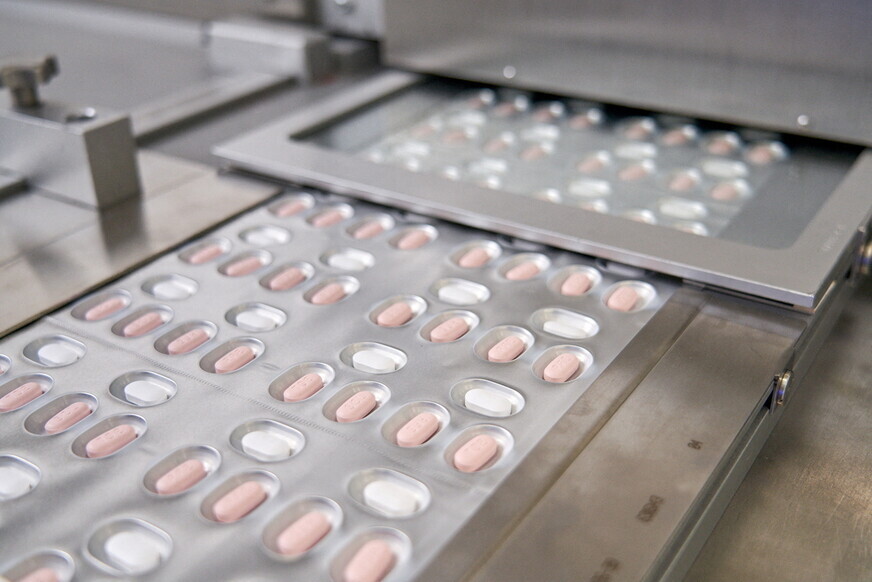
Pfizer’s oral medication for COVID-19, which has just been approved for use by the US Food and Drug Administration (FDA), is set to arrive in South Korea next week. As the world’s first treatment pill for COVID-19 that can be taken at home, rather than administered intravenously, it’s regarded as the next milestone in the battle against COVID-19 after vaccines.
The FDA announced Wednesday that it had approved Paxlovid, the antiviral pill developed by Pfizer, for emergency use at home. The pills can be taken by patients aged 12 and above who are at high risk of having a serious case of COVID-19. Patients must weigh at least 40 kilograms, or 88 pounds.
Paxlovid can only be purchased with a prescription from a physician.
In Pfizer’s clinical trials, Paxlovid was found to reduce the risk of hospitalization and death among high-risk patients by 89%.
“Today’s authorization introduces the first treatment for COVID-19 that is in the form of a pill that is taken orally,” said Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research.
“This authorization provides a new tool to combat COVID-19 at a crucial time in the pandemic as new variants emerge,” Cavazzoni said, referring to the Omicron variant of the COVID-19 virus.
Paxlovid should be taken every 12 hours for five days immediately after the onset of COVID-19 symptoms. Pfizer said it can produce 180,000 courses of 30 pills each of the medication this year and 120 million courses next year.
The US government has already contracted with Pfizer to purchase 10 million courses of Paxlovid. President Joe Biden said Wednesday that 250,000 courses would be distributed around the US in January.
Another oral antiviral for COVID-19, called molnupiravir, has been developed by Merck. In clinical experiments, molnupiravir was around 30% effective at reducing the risk of hospitalization or death among high-risk patients, which was lower than Paxlovid.
As a result, Korea’s disease control authorities have been negotiating to buy more Paxlovid, given its higher efficacy.
The Korea Disease Control and Prevention Agency (KDCA) had originally planned to make an announcement on Thursday about the schedule and volume of antiviral pills that will be arriving in Korea, but it delayed the announcement. The KDCA said it will make the announcement following the FDA’s emergency use authorization due to ongoing negotiations about accelerating the timeframe for acquiring the pills and because the volume of pills could increase by next week through additional purchase negotiations.
“We’re in deliberations with the drugmakers in question with the goal of making the pills available this year,” said Son Young-rae, director of the Central Disaster Management Headquarters’ social strategy group, in a background briefing on Dec. 2.
“We’re negotiating for more than the 70,000 courses of the Pfizer [drug that we’ve already arranged to buy]. We will provide more details about the amount we’re acquiring, the time frame, and the method of use according to the schedule of the FDA’s emergency use authorization,” Kim Ok-su, a senior official at Central Disease Control Headquarters, said on Thursday.
The government has secured around 404,000 courses of oral medication for COVID-19. It has already signed purchase contracts for 70,000 courses of Paxlovid and 242,000 courses of molnupiravir, while deliberations are ongoing for other courses.
“Since this isn’t delivered intravenously, it should be a very useful method for patients who are receiving at-home treatment. We’re planning to use this with at-home patients and at hospitals that treat high-risk patients and patients with mild and moderate symptoms,” Kim said in regard to how the pills will be used.
By Lim Jae-hee, staff reporter; Hwang Joon-bum, Washington correspondent
Please direct questions or comments to [english@hani.co.kr]

Editorial・opinion
![[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press [Editorial] Penalties for airing allegations against Korea’s first lady endanger free press](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0502/1817146398095106.jpg) [Editorial] Penalties for airing allegations against Korea’s first lady endanger free press
[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press![[Editorial] Yoon must halt procurement of SM-3 interceptor missiles [Editorial] Yoon must halt procurement of SM-3 interceptor missiles](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/child/2024/0501/17145495551605_1717145495195344.jpg) [Editorial] Yoon must halt procurement of SM-3 interceptor missiles
[Editorial] Yoon must halt procurement of SM-3 interceptor missiles- [Guest essay] Maybe Korea’s rapid population decline is an opportunity, not a crisis
- [Column] Can Yoon steer diplomacy with Russia, China back on track?
- [Column] Season 2 of special prosecutor probe may be coming to Korea soon
- [Column] Park Geun-hye déjà vu in Yoon Suk-yeol
- [Editorial] New weight of N. Korea’s nuclear threats makes dialogue all the more urgent
- [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
- [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
- [Editorial] Japan’s rewriting of history with Korea has gone too far
Most viewed articles
- 160% of young Koreans see no need to have kids after marriage
- 2[Editorial] Penalties for airing allegations against Korea’s first lady endanger free press
- 3Presidential office warns of veto in response to opposition passing special counsel probe act
- 4Hybe-Ador dispute shines light on pervasive issues behind K-pop’s tidy facade
- 5Anti-immigration candidate marauds across Korea with squad detaining foreigners
- 6Months and months of overdue wages are pushing migrant workers in Korea into debt
- 7Alleged drug use by Korean A-listers rocks nation – but not for the first time
- 8[Column] Unsettling moves by the UN Command lay way for Korean involvement in Taiwan
- 9[Reporter’s notebook] In Min’s world, she’s the artist — and NewJeans is her art
- 10Bills for Itaewon crush inquiry, special counsel probe into Marine’s death pass National Assembly