hankyoreh
Links to other country sites 다른 나라 사이트 링크
Korean researchers find drug that is more effective than remdesivir at treating COVID-19
A research team at the Institut Pasteur Korea has found a drug that appears to be much more effective than remdesivir, one of the leading candidates for inhibiting the novel coronavirus. The institute has teamed up with 10 hospitals to launch an investigator-initiated clinical trial.
“Our experiment on 24 therapeutic candidates for inhibiting the coronavirus in human lung cells found that Nafamostat, an anticoagulant that is also used to treat pancreatic cancer, was the most potent antiviral inhibitor,” said Kim Seung-taek, director of the Zoonotic Virus Lab at the Institut Pasteur Korea on May 14.
The research team posted their findings on bioRxiv, a preprint server for biological research papers, on May 12. Researchers also applied for a patent and submitted the paper to international journals.
Drawing inspiration from recent German research that found that a protease (an enzyme that breaks down proteins) called TMPRSS2 primes the spike protein that the coronavirus uses to enter cells, the team researched various drugs’ antiviral efficacy in inhibiting that protease.
After making its way into human cells by way of the spike protein on its surface, the coronavirus starts replicating itself in mass to infect the cell. Inside the cell, the coronavirus’s genomic RNA creates protease that splits the RNA into 16 pieces, including the RNA polymerase that’s in charge of replication. Inhibiting the protease or the RNA polymerase would be one way to stop the virus’s unlimited proliferation.
Remdesivir and Avigan, which are currently undergoing clinical trials, are both effective at inhibiting the RNA polymerase. Kaletra (a drug that combines lopinavir and ritonavir) is effective at inhibiting the protease.
After identifying 24 potential coronavirus medications in a drug repositioning (or repurposing) study that began in February, the team began an experiment with a Vero cell culture. Vero cells, a cell lineage that originated from the kidney of the African green monkey, are frequently used in cell cultures.
“The coronavirus causes the disease COVID-19 when it infiltrates the human lungs. Considering that the coronavirus’s infection of Vero cells differed from its infection of lung cells, we carried out an additional experiment on human lung cell cultures,” Kim explained. Researchers used lung cells from the Calu-3 lineage, which derives from lung cancer cells.
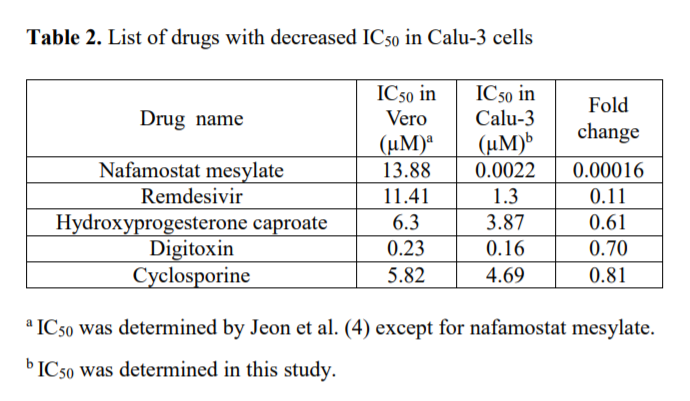
The experiment found that, while Nafamostat had little antiviral efficacy in inhibiting the coronavirus in Vero cells, it was the most potent of all the drug candidates in Calu-3 lung cells. Nafamostat’s IC50 (that is, the concentration of the substance needed to inhibit viral replication by 50%) was 13.88μm in Vero cells but 0.0022μm in lung cells. That was 600 times smaller than the 1.3μm results for remdesivir in lung cells, indicating how effective Nafamostat is against the coronavirus.
Since Nafamostat has already been approved in Korea and Japan as an anticoagulant and a pancreatitis medication, researchers can skip animal testing and the first phase of clinical testing and immediately undertake the second phase of clinical testing for coronavirus treatment. Based on these research findings, and with the approval of Korea’s Ministry of Food and Drug Safety (MFDS), the Institut Pasteur Korea is moving ahead with an investigator-initiated clinical trial involving 10 hospitals under the overall direction of Bae In-gyu, a professor of infectious disease at Gyeongsang National University Hospital. If the results of this clinical trial lead to MFDS approval, Nafamostat could be used to treat the coronavirus in Korea.
By Lee Keun-young, senior staff writer
Please direct comments or questions to [english@hani.co.kr]

Editorial・opinion
![[Column] Season 2 of special prosecutor probe may be coming to Korea soon [Column] Season 2 of special prosecutor probe may be coming to Korea soon](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0426/3317141030699447.jpg) [Column] Season 2 of special prosecutor probe may be coming to Korea soon
[Column] Season 2 of special prosecutor probe may be coming to Korea soon![[Column] Park Geun-hye déjà vu in Yoon Suk-yeol [Column] Park Geun-hye déjà vu in Yoon Suk-yeol](https://flexible.img.hani.co.kr/flexible/normal/500/300/imgdb/original/2024/0424/651713945113788.jpg) [Column] Park Geun-hye déjà vu in Yoon Suk-yeol
[Column] Park Geun-hye déjà vu in Yoon Suk-yeol- [Editorial] New weight of N. Korea’s nuclear threats makes dialogue all the more urgent
- [Guest essay] The real reason Korea’s new right wants to dub Rhee a founding father
- [Column] ‘Choson’: Is it time we start referring to N. Korea in its own terms?
- [Editorial] Japan’s rewriting of history with Korea has gone too far
- [Column] The president’s questionable capacity for dialogue
- [Column] Are chaebol firms just pizza pies for families to divvy up as they please?
- [Column] Has Korea, too, crossed the Rubicon on China?
- [Correspondent’s column] In Japan’s alliance with US, echoes of its past alliances with UK
Most viewed articles
- 1[Column] Season 2 of special prosecutor probe may be coming to Korea soon
- 2‘We must say no’: Seoul defense chief on Korean, USFK involvement in hypothetical Taiwan crisis
- 3Is N. Korea threatening to test nukes in response to possible new US-led sanctions body?
- 4Division commander ordered troops to enter raging flood waters before Marine died, survivor says
- 5Is Japan about to snatch control of Line messenger from Korea’s Naver?
- 6No good, very bad game for Korea puts it out of Olympics for first time since 1988
- 7[Editorial] Korea’s surprise Q1 growth requires objective assessment, not blind fanfare
- 8Korea’s 1.3% growth in Q1 signals ‘textbook’ return to growth, says government
- 9N. Korean delegation’s trip to Iran shows how Pyongyang is leveraging ties with Moscow
- 10Amnesty notes ‘erosion’ of freedom of expression in Korea in annual human rights report